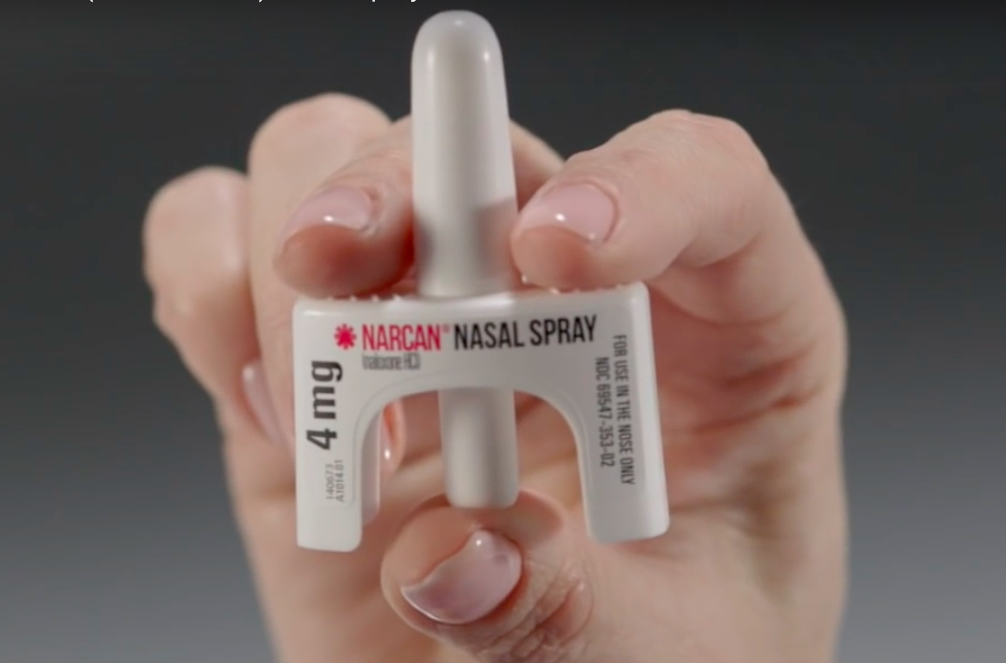
The Food and Drug Administration (FDA) has approved an extended shelf life for Narcan® (naloxone) Nasal Spray, increasing it from 24 months to 36 months. Narcan Nasal Spray is an opioid antagonist indicated for the emergency treatment of known or suspected opioid overdose, as manifested by respiratory and/or central nervous system depression. It is intended for immediate administration as emergency therapy in settings where opioids may be present. If your Narcan label says it expires in August 2020, it is now good until August 2021. Recently, the FDA recommended that healthcare professionals discuss naloxone with all patients when prescribing opioid medications. See the article here.